Dihedral Angles in Texturally Equilibrated Rocks
Solid materials
A summary of the published values for solid-solid-solid dihedral angles of geological relevance is presented in Table 4, with an indication of whether the results are the medians of populations of apparent angles measured on 2-D sections or whether they pertain to true 3-D values. Angle populations in single-phase materials have a median value of 120˚ (the only possible result when all three angles at each triple junction are included), with a range dependent on the extent of anisotropy of grain boundary energy (e.g. Kruhl, 2001; Leibl et al., 2007). For two-phase junctions, angle distributions tend to have a standard deviation in the region of 10-15˚.
Table 4. Solid-solid-solid equilibrium dihedral angles of geological relevance
| Phases | Median dihedral angle | Range of true angles | Reference |
|---|---|---|---|
| Sphalerite-galena-galena | 134° | Stanton (1964) | |
| Sphalerite-galena-galena | 111 – 128° | Lusk et al. (2002) | |
| Chalcopyrite-sphalerite-sphalerite | 130° | Stanton (1964) | |
| Pyrrohtite-sphalerite-sphalerite | 102 – 114° | Lusk et al. (2002) | |
| Galena-sphalerite-sphalerite | 133° | Stanton (1964) | |
| Galena-sphalerite-sphalerite | 27 – 111° | Lusk et al. (2002) | |
| Quartz-orthoclase-orthoclase | 52 – 149° | Vernon (1968) | |
| Quartz-plag-plag | 79 – 139° | Vernon (1968) | |
| Quartz-plag-plag | 106° | Hiraga et al. (2002) | |
| Quartz-garnet-garnet | 43 – 138° | Vernon (1968) | |
| Quartz-calcite-calcite | 68 – 146° | Vernon (1968) | |
| Quartz-apatite-apatite | 35 – 107° | Vernon (1968) | |
| Plag- quartz-quartz | 71 – 158° | Vernon (1968) | |
| Plag-quartz-quartz | 117° | Hiraga et al. (2002) | |
| Plag-hornblende-hornblende | 27 – 149° | Vernon (1968) | |
| Plag-hornblende-hornblende | 55 – 163° | Vernon (1970) | |
| Plag-augite-augite | 57 – 170° | Vernon (1968) | |
| Plag-augite-augite | 30 – 159° | Vernon (1970) | |
| Orthoclase-garnet-garnet | 38 – 132° | Vernon (1968) | |
| Garnet-quartz-quartz | 90 – 180° | Vernon (1968) | |
| Garnet-orthoclase-orthoclase | 94 – 165° | Vernon (1968) | |
| Hornblende-augite-augite | 83 – 180° | Vernon (1968) | |
| Hornblende-plag-plag | 46 – 180° | Vernon (1968) | |
| Hornblende-plag-plag | 62 – 162° | Vernon (1970) | |
| Augite-plag-plag | 72 – 150° | Vernon (1968) | |
| Augite-plag-plag | 52 – 155° | Vernon (1970) | |
| Augite-olivine-olivine | 79° | Toramaru & Fujii (1986) | |
| Augite-orthopyroxene-orthpyroxene | 86° | Toramaru & Fujii (1986) | |
| Orthopyroxene-olivine-olivine | 106 – 114° | Fujii et al. (1986) | |
| Orthopyroxene-olivine-olivine | 91° | Toramaru & Fujii (1986) | |
| Orthopyroxene-augite-augite | 113.5° | Toramaru & Fujii (1986) | |
| Olivine-orthopyroxene-orthopyroxene | 119 – 122° | Fujii et al. (1986) | |
| Olivine-orthopyroxene-orthopyroxene | 117° | Toramaru & Fujii (1986) | |
| Olivine-augite-augite | 129° | Toramaru & Fujii (1986) | |
| Ilmenite-orthoclase-orthoclase | 92 – 162° | Vernon (1968) | |
| Calcite-quartz-quartz | 98 – 152° | Vernon (1968) | |
| Apatite-quartz-quartz | 87 – 180° |
The sensitivity of equilibrium angles to pressure and temperature has been little studied. The population of true equilibrium dihedral angles in quartz aggregates (the quartz-quartz-quartz angle) is not sensitive to temperature (Kruhl, 2001), whereas the median of equilibrium two-phase (apparent) dihedral angles in sulphides (e.g. at sphalerite-galena-galena junctions) decreases significantly with increasing temperature, with potential use as a geothermometer (Stanton and Gorman, 1968; Lusk et al. 2002).
Melt-bearing materials
A summary of the published values for melt-solid-solid dihedral angles of relevance to melt-bearing rocks is given in Table 5. Most of the published measurements have been made on 2-D sections; the reported values for these studies are the medians of the measured population. Only two studies to date have reported measurements of true 3-D angles (Cmíral et al. 1998, Holness, 2006). Typical frequency distributions of the true 3-D angles are shown in Figure 3.
Table 5. Melt-solid-solid equilibrium dihedral angles of geological relevance
| solid | melt | Range of median angles | Median of true 3-D angles | source |
|---|---|---|---|---|
| quartz | Dry Silicic | 60° | Jurewicz & Watson (1984) | |
| quartz | Dry silicic | 59° | Jurewicz & Watson (1985) | |
| quartz | Hydrous silicic melt | 12 – 18° | Laporte (1994) | |
| quartz | Hydrous silicic melt | 34 – 58° | Holness (1995) | |
| quartz | rhyolite | 19°, s.d. 9.7° | Holness (2006) | |
| feldspar | Dry silicic | 44° | Jurewicz & Watson (1985) | |
| plagioclase | Anorthositic melt | 45° | Longhi & Jurewicz (1995) | |
| feldspar | silicic | 41 – 54° | Gleason et al. (1999) | |
| feldspar | silicic | 28.5°, s.d. 12.2° | Laporte & Provost (2000) | |
| plagioclase | basalt | 26°, s.d. 11.6° | Holness (2006) | |
| plagioclase | rhyolite | 24°, s.d. 11.5° | Holness (2006) | |
| plagioclase | Inninmorite (64 wt.% SiO2) | 23°, s.d. 10.2° | Holness (2006) | |
| leucite | tephrite | 20°, s.d. 10.9° | Holness (2006) | |
| silicate | Fe-Ni-S alloy | 99 – 125° | Shannon & Agee (1996) | |
| perovskite | Fe melt | 51 – 94° | Takafujii et al. (2004) | |
| perovskite | Fe-O-S liquid | 79 – 102° | Terasaki et al. (2007) | |
| perovskite | Fe-Si alloy | 130 – 140° | Mann et al. (2008) | |
| olivine | Fe-Ni-S alloy | 60 – 93° | Minarik et al. (1996) | |
| olivine | Fe-Ni-S-O melt | 96 – 106° | Holzheid et al. (2000) | |
| olivine | Fe-S alloy | 66 – 106° | Terasaki et al. (2005) | |
| olivine | Fe-O-S alloy | 54 – 98° | Terasaki et al. (2008) | |
| olivine | basalt | 47° | Waff & Bulau (1979) | |
| olivine | basalt | 41 – 47° | Fujii et al. (1986) | |
| olivine | basalt | 49° | Toramaru & Fujii (1986) | |
| olivine | basalt | 0 – 10° | Cmíral et al. (1998) | |
| olivine | basalt | 29°, s.d. 12.7° | Holness (2006) | |
| olivine | "silicate melt” (basalt°) | 0 – 45° | Yoshino et al. (2009) | |
| olivine | picrite | 29°, s.d. 9.0° | Holness (2006) | |
| olivine | carbonatite | 28° | Hunter & McKenzie (1989) | |
| olivine | carbonatite | 23 – 36° | Watson et al. (1990) | |
| olivine | carbonatite | 25 – 30° | Minarik & Watson (1995) | |
| olivine | Si-rich mantle melt | 50° | Maumus et al. (2004) | |
| olivine | phonolite | 29°, s.d. 11.9° | Holness (2006) | |
| olivine | komatiite | 32 – 33° | Jurewicz & Jurewicz (1986) | |
| olivine | komatiite | 29 – 33° | Walker et al. (1988) | |
| hornblende | Silicic melt | 25° | Laporte & Watson (1995) | |
| hornblende | Granitic melt | 53 – 58° | Lupulescu & Watson (1999) | |
| hornblende | Tonalitic melt | 46 – 48° | Lupulescu & Watson (1999) | |
| biotite | Silicic melt | 23 - 39 | Laporte & Watson (1995 | |
| augite | basalt | 98° | Toramaru & Fujii (1986) | |
| diopside | Diopside-anorthite melt | 33 – 60° | Ikeda et al. (2002) | |
| augite | Inninmorite (64 wt.% SiO2) | 38°, s.d. 13.4° | Holness (2006) | |
| augite | phonolite | 37°, s.d. 14.8° | Holness (2006) | |
| augite | basalt | 37.5°, s.d. 13.0° | Holness (2006) | |
| orthopyroxene | basalt | 52 – 70° | Fujii et al. (1986) | |
| orthopyroxene | basalt | 76° | Toramaru & Fujii (1986) | |
| orthopyroxene | basalt | 20 – 40° | Von Bargen & Waff (1988) | |
| chromite | Sulphide liquid | 41 – 53° | Brenan & Rose (2002) |
Figure 3. Texturally equilibrated melt-solid-solid dihedral angles
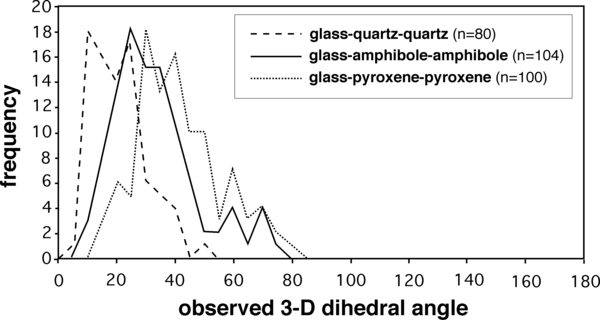
Frequency distributions of true dihedral angles measured for texturally equilibrated natural examples of rapidly quenched melt-bearing systems. From Holness (2006).
The variation of the liquid-solid-solid equilibrium dihedral angle with pressure, temperature and fluid composition is controlled by the layer of adsorbed species on the interfaces (Holness, 1993; Brenan and Rose, 2002; Takei and Shimizu, 2003). In general, the higher the extent of surface activity the more sensitive is the equilibrium angle to changes in pressure and temperature (Holness, 1993; Holness and Graham, 1995).
If the liquid has a similar composition and structure to the solid then the energy of the liquid-solid interface, and thus the dihedral angle, is low. The smallest angles are generally found in single component systems such as ice-water (Walford and Nye, 1991; although see Mader (1992)) or systems with very high solubility of the solid phase (e.g. sucrose-H2O, Pharr and Ashby, 1983; NaCl-H2O ice, Spetzler and Anderson, 1968; olivine-H2O at high pressure, Yoshino et al, 2007). In simple binaries, the dihedral angle is a function of temperature (and thus liquid composition), with the lowest angles when the liquid composition is closest to that of the solid phase (Takei, 2000; Ikeda et al., 2002; Takei and Shimizu, 2003).
Conversely, the highest angles occur where the solid and liquid have the most disparate compositions and structures. Examples of the latter include argon-calcite, argon-quartz, CO2-quartz (Holness 1993), metal-olivine (see list of references in Table 5). Fe-S liquids in silicate matrices also have generally high dihedral angles (e.g. Ballhaus and Ellis, 1996; Minarik et al., 1996; Gaetani and Grove, 1999; Terasaki et al., 2007; again see Table 5 for a more comprehensive list) although these are sensitive to changes in oxygen fugacity and pressure (Gaetani and Grove, 1999; Rose and Brenan, 2001; Takafuji et al., 2004; Terasaki et al. 2008).