Mineral chemistry
In selected samples of metadolerites (samples MC70, MC77, MC81; Table 1) electron microprobe analyses were carried out on relict igneous clinopyroxene and metamorphic minerals, as amphibole, chlorite, pumpellyite, and white mica. The results are reported in Tables 3, 4, 5.
Clinopyroxene
Structural formula of clinopyroxene was calculated on the basis of 6 oxygens and classified by using the pyroxene nomenclature suggested by Morimoto (1988, 1989); the chemical compositions of clinopyroxene are reported in Table 3. Clinopyroxene is enriched in augite and augite-diopside components and displays high Al and Fe contents (Al2O3= 4.95-6.86 wt %; FeO=6.05-18.80 wt %; Table 3). The only exception is represented by acicular clinopyroxene crystals with aegirine-augite composition (e.g. Na2O=6.90 wt %; Table 3, sample MC81-21; see also Fig. 7a).
Amphiboles
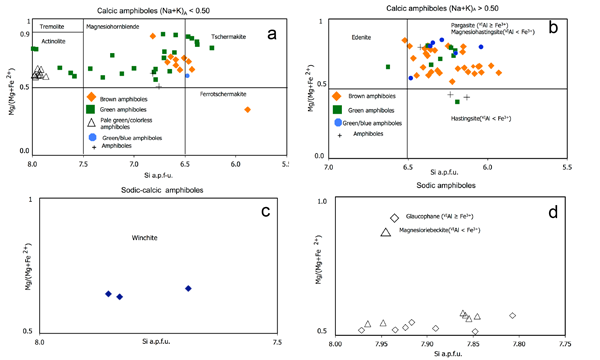
Structural formula of amphibole was recalculated on the basis of 23 oxygens and classified by using the amphiboles nomenclature suggested by Leake et al. (1997, 2004). The chemical compositions of the analysed amphiboles are reported in Table 4. They include Ca-amphiboles (Figs. 6a, 6b), Na-Ca-amphiboles (Fig. 6c), and Na-amphiboles (Fig. 6d). The analysed amphiboles are: brown, green, pale-green/colorless, blue, and blue-green amphiboles.
Brown amphiboles with (Na+K)A<0.50 have magnesiohornblende and ferrotschermakite compositions (Fig. 6a). Brown amphiboles with (Na+K)A≥0.50 and Ti<0.50 show magnesiohastingsite, edenite, and pargasite compositions (Fig. 6b). Green and pale green/uncoloured amphiboles with (Na+K)A<0.50 show magnesiohornblende, actinolite, and tschermakite compositions (Fig. 6a), whilst green amphiboles with (Na+K)A≥0.50 and Ti<0.50 show magnesiohastingsite, hastingsite, and edenite compositions (Fig. 6b). The blue-green amphiboles with (Na+K)A≥0.50 and Ti< 0.50 have magnesiohastingsite composition, while those with (Na+K)A<0.50 show a tschermakite composition. The sodic-calcic amphibole shows a winchite composition (Fig. 6c): it occurs at the rim of brown amphibole and is in turn rimmed by blue amphibole corona. Blue amphibole exhibits glaucophane and magnesioriebeckite compositions (Fig. 6d).
Figure 7. Textural features and microchemical composition of metadolerite MC81.
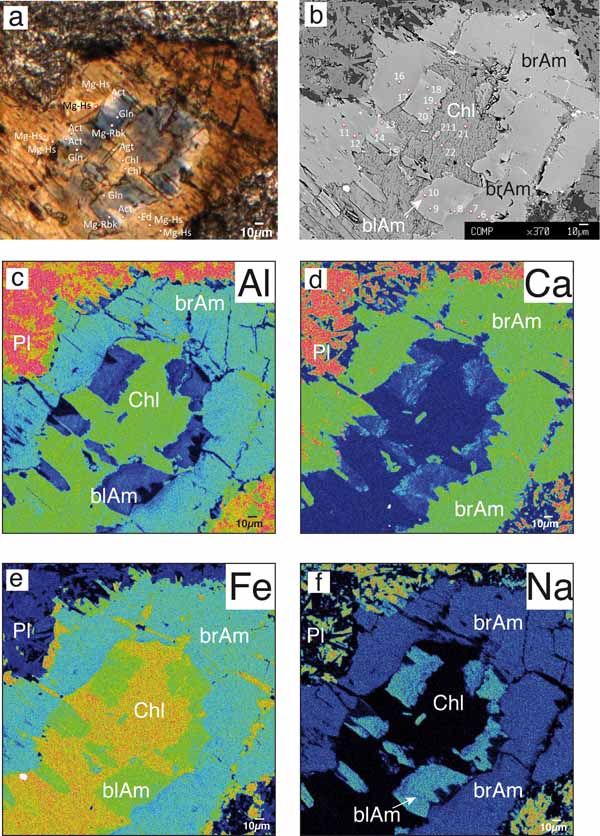
a) Photomicrograph of a selected microstructural site showing amphiboles replacing magmatic clinopyroxene (middle) and altered plagioclase (upper side); plane polarized light. The shape of magmatic clinopyroxene (now replaced by brown amphibole) is still recognizable. Brown amphibole is rimmed by uncoloured and blue amphiboles growing towards the core of the microstructural site. Location of the analysed spots is also indicated. b) BSE image of the same sample portion with location of the analysed spots (analysis number as in Table 4). c-f): elemental maps relative to Al, Ca, Fe and Na distribution, respectively.
One sample (MC81; Table 1) was selected for showing microstructural domains characterized by pseudomorphic and coronitic textures. Microchemical composition and location of the analysed spots are reported in Figures 7a and 7b, and in Table 4. In such domains, brown amphibole (Mg-hastingsite) is pseudomorphic after clinopyroxene (replacive amphibole) and is rimmed by pale green/uncoloured amphibole (actinolite). The brown and pale green amphiboles are rimmed by blue amphibole (glaucophane and Mg-riebekite). The core of these domains consists of chlorite + aegirin-augite (Fig. 7a) which probably replaced magmatic clinopyroxene. Detailed microchemical analyses and elemental maps were performed on these domains as illustrated in Figures 7c-7f. The elemental maps clearly show that Al and Ca decrease from the brown to the blue amphibole (Figs. 7c and 7d), whilst Na and Fe increase from the brown amphibole to the blue amphibole (Figs. 7e and 7f).
Chlorite
Chlorite was recalculated on the basis of 28 oxygens. Selected analyses are reported in Table 5. Chlorite is classified according with Hey (1954)’s nomenclature. The analysed chlorite occurs at the core of textural sites near blue amphibole rimming brown amphibole (see Figs. 7a, 7b; analyses 21 and 22 in Table 5) and in veins associated with colourless amphibole (analysis 160 in Table 5).
Pumpellyite
Pumpellyite occurs in veins and as pseudomorph after magmatic plagioclase. Pumpellyite composition is characterized by relatively high content of Al (Al2O3=22.68-26.04 wt %) and Fe (FeO=2.83-7.43 wt %); MgO content is between 2.31-3.43 wt %. Pumpellyite shows a linear and negative Fetot vs Al2O3 .
White mica
The structural formula of white mica was recalculated on the basis 11 oxygens. Using the calculations suggested by Bousquet et al. (2002), white mica in the Frido Unit metadolerites shows a phengitic composition (Si = 3.133-3.425 a.p.f.u.).