There are many stages of wall rock alteration and the main type is green rock with characteristic minerals assemblage of epidote- actinolite-chlorite-carbonate. There are different altered rocks developed such as pyrite sericite quartz rock and sericitization. The sequence of wall rock alteration as follows: epidote- actinolite- chlorite; carbonate- sericite quartz rock-silicification, then later carbonate. There exists alteration zone from wall rock to ore body: chlorite→actinolite→pyrite sericite quartz rock; and there are follow altered rock zoning for the biotite-plageclase granulite: fading biotite →hydromica→pyrite sericite quartz rock→silicification. The petrochemical equilibrium calculation of each alteration rocks (Table 1) shows that the mineralizing fluids enter wall rock along the fault structure and the following components such as SiO2, Fe2O3, K2O,Na2O and H2O were added, but the components such as Al2O3, TiO2, FeO, CaO, MgO were leaching loss. The main alteration minerals are chlorite and mica, in order to research the relation between the alteration and mineralization, the characteristics of these two minerals have been studied in detail. Many analytic methods such as electron probe analysis, X-ray diffraction, differential analysis, IR spectrum have been used.
Table 1. Petrochemical equilibrium calculation of alteration rocks in Caijiaying Pb-Zn-Ag deposit
| oxidate | biotite-plageclase amphibolite | actinolite | pyrite sericite quartz rock | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| wt% | cation molecule | unit cell | ion number | wB% | cation molecule | ion number | carry out numbers of unit cell | carry out numbers of ion | wt% | cation molecule | ion number | carry out numbers of unit cell | carry out numbers of ion | |
| SiO2 | 51 | 850 | 850 | 466.8 | 59.48 | 991.33 | 991.33 | 487.71 | 20.91 | 57.23 | 953.83 | 953.83 | 471.61 | 4.81 |
| Al2O3 | 16.24 | 159.22 | 318.44 | 174.88 | 16.57 | 162.45 | 324.9 | 159.84 | -15.04 | 8.27 | 81.08 | 162.16 | 80.18 | -94.7 |
| TiO2 | 1.66 | 20.8 | 20.8 | 11.42 | 0.66 | 8.27 | 8.27 | 4.07 | -7.35 | 0.51 | 6.39 | 6.39 | 3.16 | -8.26 |
| Fe2O3 | 4.5 | 28.19 | 56.39 | 30.97 | 6.55 | 41.04 | 82.08 | 40.38 | 9.41 | 9.17 | 57.46 | 114.91 | 56.82 | 25.85 |
| FeO | 6.14 | 85.51 | 85.51 | 46.96 | 1.17 | 16.29 | 16.29 | 8.01 | -38.95 | 1.55 | 21.59 | 21.59 | 10.67 | -36.29 |
| CaO | 5.87 | 104.82 | 104.82 | 57.56 | 0.8 | 14.29 | 14.29 | 7.03 | -50.53 | 2.59 | 46.25 | 46.25 | 22.87 | -34.69 |
| MgO | 3.56 | 88.56 | 88.56 | 48.64 | 1.18 | 29.36 | 29.36 | 14.44 | -34.2 | 2.11 | 52.49 | 52.49 | 25.95 | -22.69 |
| K2O | 4.14 | 43.59 | 87.9 | 48.27 | 4.84 | 51.38 | 102.76 | 50.56 | 2.29 | 3.82 | 40.55 | 81.1 | 40.1 | -8.17 |
| Na2O | 2.9 | 46.82 | 91.2 | 50.09 | 0.15 | 2.43 | 4.85 | 2.39 | -47.7 | 2.35 | 37.96 | 75.93 | 37.54 | -12.55 |
| P2O5 | 1.18 | 8.32 | 16.64 | 9.14 | 1.58 | 11.14 | 22.28 | 10.96 | 1.82 | 3.39 | 23.89 | 47.78 | 23.62 | 14.48 |
| MnO | 0.15 | 2.12 | 2.12 | 1.16 | 0.06 | 0.84 | 0.84 | 0.41 | -0.75 | 0.11 | 1.55 | 1.55 | 0.77 | -0.39 |
| CO2 | 1.22 | 27.73 | 27.73 | 15.23 | 0.54 | 12.28 | 12.28 | 6.04 | -9.19 | 2.85 | 64.78 | 64.78 | 32.03 | 16.8 |
| H2O+ | 1.27 | 70.56 | 141.11 | 77.49 | 6.26 | 347.78 | 695.56 | 342.2 | 264.71 | 8.97 | 498.33 | 996.67 | 492.79 | 415.3 |
Based on the electron probe analysis from 18 chlorite samples, the crystal chemical formula have been calculated and then ploting them on the genesis figure, they can be divided into following type: the main type is brunsvigite, the others are prochlorite, chamosite and diabantite. The typical characteristics of chlorites in the Caijiaying deposit are iron rich higher than the general hydrothermal poly metal deposits.
The analytic results of IR spectrum for chlorites listed in Table 2). Two stronger dilation vibration zone of OH- located at near 3556~3538cm-1 and 3427~3390cm-1, at the same time, dilation vibration zone of Si-O-Si(Al) is a very stronger single peak with lower and mostly at near 990cm-1. According to the classification by Menka (Ying Y P, 1982), the chlorites in Caijiaying deposit can be classified as two types, and most of them are thuringite, the others are ripidolite. The other OH- dilation vibrated frequency locates between 3430~3390cm-1; a few samples are ripidolite whose librated zone of OH- locates at 3560cm-1, 3425cm-1 and bend librated at near 655cm-1.
Table 2. The vibration frequency of infrared spectra of chlorite in the Caijiaying mineral district (cm-1)
| No. | sample position | νOH | νM-OH | νSi-O-Si(Al) | σSi-O-Si(Al) | name |
|---|---|---|---|---|---|---|
| 1 | Zk315-30 | 35,533,415 | 665,623 | 996,816 | thuringite | |
| 2 | Zk317-3 | 35,483,391 | 666,619(sh) | 995 | thuringite | |
| 3 | Zk317-3 | 35,483,391 | 666,619(sh) | 995 | thuringite | |
| 4 | Zk315-27 | 35,463,397 | 656,626 | 984 | 748 | thuringite |
| 5 | Zk307-18 | 35,423,391 | 666,626 | 985 | 808,751 | thuringite |
| 6 | Zk307-18 | 35,383,397 | 666,625 | 990 | 775 | thuringite |
| 7 | Zk307-18 | 35,403,391 | 666,628 | 991 | 771 | thuringite |
| 8 | Zk307-24 | 35,523,379 | 666,630 | 992 | 751 | thuringite |
| 9 | Zk317-3 | 35,513,427 | 670,625 | thuringite | ||
| 10 | stratumG3 | 35,553,417 | 669,636 | 988 | 829 | thuringite |
| 11 | stratumG4 | 35,413,403 | 670,629 | 990 | 796,749 | thuringite |
| 12 | Zk315-23 | 35,583,435 | 650 | 994 | 743 | ripidolite |
| 13 | Zk311-21 | 361,635,433,441 | 662 | 990 | 815,764 | ripidolite |
| 14 | Xiaobazi bore drill | 3560 | 630 | 994 | ripidolite | |
| 15 | Zk315-23 | 35,963,296 | 662 | 1006 | 772 | chlorite |
The results of differential analysis of chlorites in the deposit listed in Table 3. Most of chlorites have two decalescence valley, located at the range 500~600oC and 600~700 oC or 700~800 oC. Based on the classification principle on the structural decompose by Werner Smyktar Kloss, the chlorites in the ore field are maily ripidolite, brunsvigite and some chlorite with deaquation and prochlorite. All those characteristics are consistent with the conclusion that chlorite often belong to brunsvigite in the Pb-Zn deposit (Ye D N, 1984).
Table 3. The breakdown temperature of chlorite structure and nomenclature
| structural decompose temperature oC | name |
|---|---|
| 580,710 | ripidolite - brunsvigite |
| 575,690 | ripidolite |
| 580,715,760 | ripidolite?brunsvigite |
| 580,743 | ripidolite?brunsvigite |
| 550 | ripidolite |
| 525,830 | ripidolite, Zn-bearing penninite |
| 520,640 | ripidolite |
| 672 | chlorite with deaquation |
| 575,780 | ripidolite,prochlorite |
| 575,755 | ripidolite,Zn-bearing penninite |
| 570,720 | ripidolite?brunsvigite |
| 540,760 | ripidolite, prochlorite |
| 570,690 | ripidolite |
According to the data of x-ray diffraction analysis on chlorites, the d(001), d(002),d(060) can be obtained, on the basis of these information, the atom numbers of Fe2+, Fe3+, Mg2+ and Si4+ in chlorites can be calculated and then plotting the results on the figure provided by Brown B E,1962, the poly type of chlorites in Caijiaying deposit are Iba, Ibb and IIbb (Table 4).
Table 4. X-ray diffraction analyses of chlorite in Caijiaying(x0.1nm)
| No. | d(002) | d(060) | poly type |
|---|---|---|---|
| 1 | 6.9919 | 1.4968 | Ibb |
| 2 | 7.3022 | 1.5395 | Iba |
| 3 | 7.2544 | 1.5607 | Ibb |
| 4 | 7.0139 | 1.545 | IIbb |
| 5 | 6.9699 | 1.545 | Iba |
| 6 | 6.9699 | 1.545 | Iba |
| 7 | 7.0251 | 1.5465 | Ibb |
| 8 | 6.9699 | 1.5465 | IIbb |
| 9 | 6.9157 | 1.5488 | IIbb |
| 10 | 7.3132 | 1.5465 | IIbb |
| 11 | 7.1725 | 1.5371 | IIbb |
Sericite is one of the main wall rock alterations in Caijiaying deposit. The experiments was finished at electron probe room of geology department, Beijing University, the results of two main ore belts and stratum are listed in Table 5, it shows that the K2O is very high, so they can be named as potassium mica. The metal oxide amounts are follows: w SiO2 (wt%) 43.20%-52.69%, w (K2O) 6.49%-10.63%, w (MgO) 0.98%-2.39%, w (FeO) 1.56%-8.77%, w (Al203) 30.47%-37.41% and minor Na2O, MnO2, TiO2. Accepted chemical computation method (Xiao P, 2001), the potassium mica chemical formula are calculated and results listed in Table 6.
Table 5. Chemical composition of potash mica analyse by electronic probe
| No. | sample position | K2O | Na2O | CaO | MgO | TiO2 | Al2O3 | MnO | SiO2 | FeO | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | V ore belt | 10.63 | 0.07 | 0.08 | 1.02 | 0 | 31.43 | 0 | 47.51 | 1.69 | 92.52 |
| 2 | V ore belt | 6.49 | 0.05 | 0.17 | 2.39 | 0 | 30.89 | 0 | 52.69 | 1.97 | 94.59 |
| 3 | III ore belt | 7.78 | 0.16 | 0.02 | 4.03 | 0.03 | 30.46 | 0.26 | 43.2 | 8.77 | 94.89 |
| 4 | III ore belt | 9.44 | 0.3 | 0.06 | 1.02 | 0 | 30.37 | 0.04 | 51.79 | 1.72 | 94.76 |
| 5 | III ore belt | 8.17 | 0.1 | 0 | 1.47 | 0.07 | 35.05 | 0.04 | 49.89 | 1.56 | 96.36 |
| 6 | V ore belt | 8.42 | 0.32 | 0.04 | 0.98 | 0.23 | 37.41 | 0.02 | 44.93 | 2.27 | 95.08 |
Table 6. Calculated crystal chemical formula (potash mica)
| No. | crystal chemical formula |
|---|---|
| 1 | K0.89(AlVI 1.71Mg0.12Fe2+ 0.08)1.91[(Si3.20AlIV 1.71)4.00O10](OH)2 |
| 2 | K0.54(AlVI 1.65Mg0.23Fe2+ 0.11)1.99[(Si3.36AlIV 0.64)4.00O10](OH)2 |
| 3 | K0.58(AlVI 1.38Mg0.22Fe2+ 0.43)2.03[(Si3.18AlIV 0.82)4.00O10](OH)2 |
| 4 | K0.80(AlVI 1.79Mg0.12Fe2+ 0.08)1.97[(Si3.21AlIV 0.79)4.00O10](OH)2 |
| 5 | K0.70(AlVI 1.84Mg0.13Fe2+ 0.08)2.07[(Si3.21AlIV 0.79)4.00O10](OH)2 |
| 6 | (K0.71Na0.08)0.79(AlVI 1.84Fe3+ 0.08Mg0.08)2.00[(Si2.99AlIV 1.01)4.00O10](OH)2 |
In order to discuss the composition variation of potassium mica, we researched from several aspects:
(i) Genesis diagram
Put the data of x(AlIV), x(Fe3+)/x(Fe3++AlIV) and x from Table 6 on the genesis diagram, all the potash mica fell in the hompgeneity multiple facial hydrothermal area of 1 Md, lM, 2M, 2M1, 2M2, 3T. This demonstrates that potassium mica is hydrothermal genesis.
Figure 2. Characteristics of crystal chemistry of dioctahedron sericites in different genesis
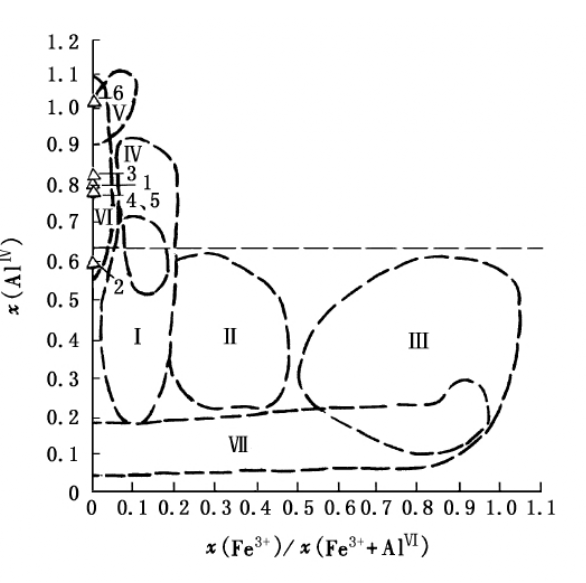
I~III. Diagenesis – initial epigenesis 1 Md-1 M polyphase of sedimentary mica; I. Al illite area; II. evaporation of illite; III1 glauconite; IV. 1M2 M1 polyphase of mica in deep epigenesis belt ;V. metamorphic belt of mica; Ⅵ.1 Md, 1M, 2M1, 2M2 and 3T polyphase hydrothermal mica; VII. 1 pictoamesite area(after A T Kososyvier and B.A. Deliz,1975)
(ii) The relation of K and Na in potassium
The K ion number of potassium mica in Caijiaying ore body changes between 0.54 and 0.89, but Na ion number is 0. Though the K ion number of potassium mica in the stratum of Datongying Formation is 0.71 which is near to the ore body, the Na is 0.08 which is different from the ore body. The reason is that Na is carried out in the rock when hydrothermal alteration occurred.
(iii) The relation of Mg and Si in potassium mica
The Mg ion number of potassium mica in Caijiaying ore body changes between 0.12 and 0.23, all Si ion number are higher than 3.00 and range from 3.18 to 3.41, they belong to silicon poly mica. But the Mg ion number in stratum is lower and Si ion number equal to 2.99 which similar to silicon poly mica.
(iv) The Al occupy position in potassium mica
The AlIV and AlVI in the tetrahedron position of the potassium mica in the ore body are 0.64-0.82 and 1.38-1.84, but the numbers that of stratum is 1.01 and 1.84 respectively. The reason may be that AlVI was carried out during hydrothermal alteration.
(v) The relation among Al, Fe, Mg in octahedron position
Plotting the Al, Fe and Mg atom numbers in octahedron position of potassium mica on the Al-Fe-Mg diagram, w(FeO),w(MgO) of sample No. 3 are the highest, 4.03% and 8.77% respectively, Fe, Mg atom numbers are 0.43 and 0.22. It demonstrates there is positive relation between Mg and Fe in octahedron position of potassium mica, w(Mg) and w(Fe) are higher, so the AlVI is lower. The other samples locate in a small area. All these characteristics show that w(A1), w(Mg) and w(Fe) are similar in potassium mica.
The nine samples of potassium mica in the deposit have been analyzed at differential analysis room of geology department, Beijing University. The structural decompose of potassium mica range from 615–860oC and most of them at 620-750oC. Based on the classification principle on the structural decompose, the potassium mica in the ore field are mainly 1M, 1M-2M1 and 2M1 poly type. The species of potassium mica can be named as sericite, hydromuscovite, hydromuscovite-sericite. The weight loss amount of potassium mica is different from 5% to 9.25%, these results are consistent with the fugitive constituent analyzed by electron probe analysis from 3.46% to 7.42%.
Ten samples of potassium mica in the deposit have been analyzed by x-ray diffraction. The experiment was finished at X-ray room of geology department, Beijing University. The crystal indexes of potassium mica are calculated and compare with the standard data by Ye D N (1984). There are different d(060) in different poly type potassium mica, the d(060) of dioctahedral and trioctahedral are 0.1480-0.1510 nm and 0.1530-0.1557 nm respectively. There are poly type of potassium mica 1M, 1M + 2M1, 2M1, 2M1 + 3T in the Caijiaying deposit (Table 7) and this result consistent with differential analysis. At the same time, there are some relation between the poly type of potassium mica and depth which they locate, and 1M poly type can be seen at 1432.6 m reflecting that the depth is shallow. But the 2M1 poly type formed at deep, 1361-1153.0 m, 1M +2M, poly type formed at 1361 and 1302 m.
Table 7. Results of x-ray diffraction potash mica
| No. | Sample location | elevation /m | poly type |
|---|---|---|---|
| 1 | V ore belt | 1433 | 1M |
| 2 | V ore belt | 1361 | 2M1 |
| 3 | V ore belt | 1361 | 1M+2M1 |
| 4 | III ore belt | 1357 | 2M1 |
| 5 | V ore belt | 1317 | 2M1 |
| 6 | V ore belt | 1302 | 1M+2M1 |
| 7 | V ore belt | 1230 | 2M1 |
| 8 | III ore belt | 1153 | 2M1 |
| 9 | III ore belt | 1128 | 2M1 |
| 10 | III ore belt | 1121 | 2M1+3T |
There exists positive linear correlation between Fe2++AlIV and Fe/(Fe+Mg) in the chlorites from hydrothermal poly metal deposits and the ratios are different ore districts. The values of AlIV and AlVI of the chlorites in Caijiaying Pb-Zn-Ag deposit are distinguished from the other types of deposits and this demonstrates that this is uniqueness. The chemical composition of chlorites is controlled by the forming environments such as temperature, pressure and acidity, etc. The acidity has affected the composition of minerals during the medium temperature hydrothermal deposits. According to the replacement principle of acid and alkali by Korzhinskii, it is favor to acid components to replace the alkali on the condition of acidity increasing of the solution. The coordination numbers of Fe2+ and Mg2+ in chlorites are usually six and thus they often replace each other. The chlorites formed in relative acid environment when Fe replaces Mg; otherwise, it formed alkali environment when Mg replaces Fe. The characteristics of chlorites in Caijiaying are that they are brunsvigite demonstrating they formed in acid environment.
The characteristics of mica is higher K2O and typical series of potassium mica, this is seldom seen at the same genesis of Pb-Zn deoposit. The data of X(AlIV)-X(Fe3+)/X (Fe3+ + AlVI) reflects that it is hydrothermal genesis.
The difference in chemical composition of stratum and ore body and shows in the correlation of Mg, Si and octahedral. But there is no difference of Fe, Mg and Al in octahedral potassium mica. The potassium mica can came from hydrothermal alteration by albite, plageclase feldspar, potash feldspar from the optical microscope obseravation. Silicication of companied with potassium mica, and pyrite to form pyrite- sericite-quartz rock which have close relation with mineralization.
There are poly types of potassium mica 1M, 1M + 2M1, 2M1, 2M1 + 3T in the Caijiaying deposit and 1Md exists in the peripheral area. At the same time, there are some relation between the poly type of potassium mica and depth which they locate, and 1M poly type can be seen at 1432.6 m reflecting that the depth is shallow. But the 2M1 poly type formed at deep, 1361-1153.0 m, 1M + 2M, poly type formed at 1361 and 1302m. This conclusion is consistent with viewpoint of Ye D N (1984) that the mica class is octahedron in Pb-Zn deposit. Pyrite sericite quartz rock in the wall rock alteration has close relation with mineralization in silver deposit and illite-hydromuscovite alteration played the second role in mineralization (Xiao P, 2001). It has very important significance to look for the same genesis deposit in the world that using the regularity that the poly type of potassium mica change with the depth.