Metamorphic reactions
In order to clarify the protolith characteristics, we considered conventional metamorphic reactions. Bulk chemistry of detrital sediments is primarily controlled by the composition of the source (e.g., Bhatia, 1983). Al2O3 or Fe2O3* contents of the Shimozaisho Group are relatively high compared to the average pelite of Shaw (1956) (Table 1). The Al- or Fe-rich composition is probably related to weathering processes (hydrolysis, oxidation, hydration, salinization) on the provenance (Retallack, 2001). The chemical compositions of the Al-rich metaclastic rocks of the Shimozaisho Group are comparable with pyrophyllite-muscovite rock of lateritic soil parentage from Central India (Sharma, 1979). However, the protoliths of the Al-rich metaclastic rocks in the Shimozaisho Group were not soils. The presence of detrital grains and sedimentary structures obviously indicate that the protolith was formed as a result of transport and redeposition deposition of detritus, which may have been derived from weathered soil. To evaluate soil component on the provenance, we attempt to identify the detrital minerals derived from the weathered soils adopting conventional metamorphic reactions as described below (Fig. 6).
Figure 6. Major sericite and chloritoid forming metamorphic reactions in the Al- and Fe-rich pelitic rocks
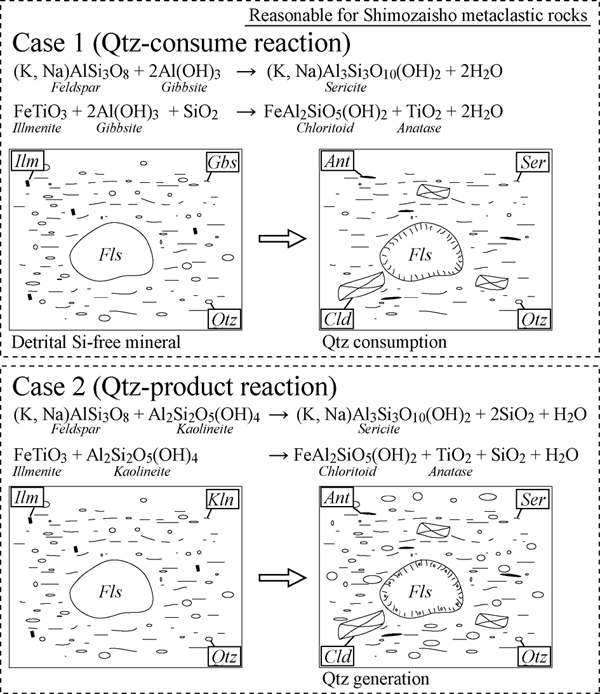
Our study shows that the Shimozaisho metaclastic rocks contain no metamorphic quartz, indicating that detrital Al-free minerals were abundant in the protolith (Case 1 is reasonable). Abbreviations: Qtz = quartz, Cld = chloritoid, Ser = sericite, Fls = feldspar, Gib = gibbsite, Kln = kaolinite, Ilm = ilmenite, Ant = anatase.
Feldspar + Gibbsite = Sericite + 2 H2O (1)
Ilmenite + 2Gibbsite + Quartz = Chloritoid + Anatase + 2 H2O (2)
We use Al-containing minerals, because the Al contents in this system is related to the amount of feldspar destroyed during weathering, which is prevailing in most types of rocks (Nesbitt and Young, 1982).
Reaction rims surrounding detrital feldspar and felsic volcanic fragment are composed of dominant sericite and no quartz (Figs. 4E, 4F) suggesting that the feldspar consumption reaction generates only sericite and no quartz (reaction (1)). The matrix is composed mainly of sericite and lesser amount of quartz (Figs. 4B, 4C, 4D, 4E) suggesting that the quartz consumption reaction (2) occurred. Occurrences of Ti-minerals suggest that ilmenite is a pre-metamorphic mineral and that anatase is a metamorphic mineral (Figs. 4F, 4G). This indicates that anatase was formed from breakdown of detrital ilmenite (reaction (2)). These observations suggest that Si-free minerals such as gibbsite were present in the protolith of the Al-rich metaclastic rocks and negate the possibility of contribution of Si-bearing minerals, such as kaolinite and halloysite, to the metamorphic reaction (Fig. 6).