Wilde, A., Simpson, L. and Hanna, S. 2002. Preliminary study of Cenozoic hydrothermal alteration and platinum deposition in the Oman Ophiolite. In: Jessell, M. J. 2002. General Contributions: 2002. Journal of the Virtual Explorer, 6, 7-13.
Geochemical Modelling (continued)
Results
One hundred grams of the model serpentinite was titrated into the four water compositions tabulated in Table 6. Despite the large differences in ionic strength and cation ratios the resultant mineralogies are remarkably similar. For example, all “altered” serpentinites are dominated by antigorite, brucite and hematite. Minor (< 1% by mass) phases include andradite and calcite (meteoric waters), phlogopite and calcite (bittern) and dolomite (seawater).
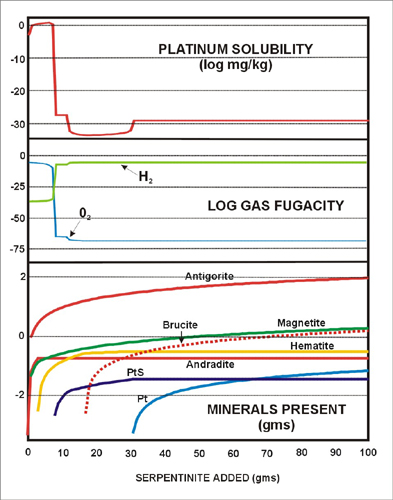
Platinum solubility is extremely high (over 1 mg/kg) in the early part of the reaction paths as oxygen fugacity remains high (Fig. 6). Platinum solubility is accounted for by a hydroxyl complex equivalent to Pt(OH)2(aq). As more of the rock is titrated into the fluid it rapidly reduces the fluid and the platinum concentration drops off dramatically. The reaction is:
Pt(OH)2(aq) —> 2H2O + 0.5O2 + Pt (1)
| Figure 6: Titration of model serpentinite into modern-day hyperalkaline groundwater . This model illustrates the dependence of Pt solubility on oxidation state. Compare this model to the more realistic flush model in Fig. 7. |
Thus, oxidation state and high pH seem to be more important factors in mobilizing platinum than the considerable variation in water composition.
We carried out a number of flush models at 35¼C both at high porosity and at low porosity, using the four water compositions described above. For high porosity models, the rock mass was set at 100kg and the notional pore space was occupied by 1.2 kg of fluid. Then 100 kg of fluid was flushed through displacing an equivalent mass of fluid from the previous step. For the low porosity step a rock mass of 10 kg was used and all other parameters were the same. Thus, the integrated fluid:rock was between one and two orders of magnitude higher (10:1 by mass) than the instantaneous fluid:rock (1:10 or 1:100).
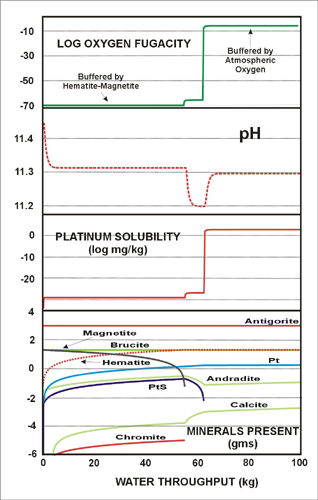
Flushing the four potential source rocks (Table 7) with the four oxidized waters (Table 6) resulted in a “spike” in fluid Pt concentration (> 1 mg/kg) in most cases, because in the early stages of reaction progress oxidation state is buffered by various minerals (namely magnetite, chromite, pyrite) and solubility remains low. As the oxidized fluid consumes the reductant minerals, oxidation state approaches the original atmospheric condition and Pt solubility increases dramatically. The fluid remains undersaturated in Pt thus dissolving all Pt present in the rock, although Pt concentrations vary slightly between models due to variations in pH. The drop off in fluid Pt content is due to replacement by the incoming oxidized but Pt-poor fluid. In the case of the chromitite, however, reaction lead to a much larger drop in pH to 7.5 and lower oxidation state. This in turn meant that the fluid was undersaturated in Pt and consequently Pt in the fluid was buffered by the presence of Pt metal (at about 0.45 mg/kg). Increasing the amount of Pt in the rock means that not all Pt is dissolved instantaneously and the Pt concentration of the fluid is relatively constant as demonstrated by Fig. 7.
| Figure 7: Flush model of modern-day hyperalkaline groundwater (with oxidation state initially buffered by atmospheric oxygen) through model serpentinite. The key insight from this model is that the ability to leach Pt is governed by the presence of magnetite. The oxidation state of the fluid is buffered at a level much lower than that required to promote solubility of native Pt as Pt hydroxide complexes, until all magnetite is destroyed, after about 50 kg of fluid has passed through the rock (i.e. integrated fluid:rock mass ratio of over 20:1). |
We conclude that providing the incoming fluid is very oxidized Pt will be easily removed from serpentinite and gabbro, regardless of fluid composition. It would take a vastly greater amount of fluid to leach Pt from the chromitite, however, because of the greater abundance of reductant minerals and pH buffering to near neutral. We have taken no account of kinetic factors of course. The rapid Pt “spike” predicted in serpentinite and gabbro assumes rapid dissolution of Pt with respect to flow rate and a network of fractures such that a transient fluid is able to come into contact with the Pt (and assuming diffusion is neglible).
Depositional mechanisms were investigated by titrating various substances into a platiniferous water equilibrated with chromitite at the higher porosity. Adding CO2 gas resulted in the generation of a magnesite and Cr oxide rock. No Pt was precipitated, as oxidation state remained high. Adding an equal mass of CH4 resulted in complete extraction of Pt from the fluid and generation of magnesite, brucite, carbon, magnesiochromite and minor dolomite and antigorite. The Pt grade of this rock is predicted to be 450 ppb. The mechanism of precipitation of Pt is clearly reduction, with oxygen fugacity falling over 60 orders of magnitude during the reaction path. Titrating 10 grams of serpentinite resulted in extraction of some Pt resulting in a grade of about 50 ppb, with an antigorite-hematite assemblage. About 10 ppm Cr was added to the rock.
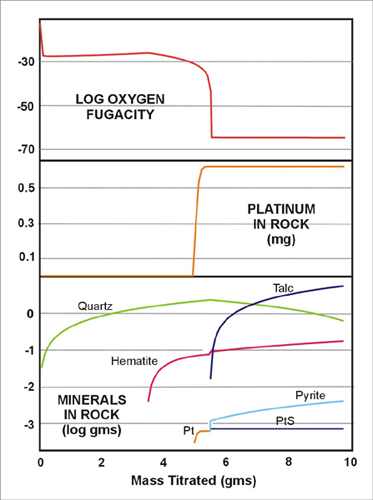
Removing Cr from the incoming fluid has a significant effect on the resulting rock. The stable assemblage is quartz, hematite and Pt phases similar to the observed SIH alteration with talc and pyrite formed at later stages of fluid rock reaction (Fig 8). Extremely large fluid:rock ratios are required for the formation of the SIH assemblage (5 gms rock:1 kg fluid). This is consistent with the abundant evidence of fracturing in the SIH serpentinite and evidence of mass gain from the bulk chemical analyses.
| Figure 8: Titration of 10 grams of unaltered serpentinite into 1 kg of “platiniferous” fluid (interacted with platiniferous serpentinite). The SIH “listwaenite” assemblage is reproduced at relatively high fluid:rock ratios. |