Preliminary
study of Cenozoic hydrothermal alteration and platinum deposition in the
Oman Ophiolite
Geochemical Modelling
Software & Computational Methods
A series of chemical models were performed using the software Geochemist’s Workbench (Bethke, 1996, herein referred to as GWB) to investigate whether various waters could mobilize ore-forming amounts of Pt from various possible source rocks and what were the likely depositional mechanisms. These took two forms: titration and flush models (see Bethke, 1996). The former simulates the addition of increments of rock to a fixed mass of hydrothermal solution, the reverse of what geologists usually consider to be the case in fluid-rock interaction. We need to differentiate between integrated fluid:rock representing the total fluid flux over the life of the hydrothermal system and the instantaneous fluid:rock representing the volume ratio of fluid to rock at a moment in time, controlled by porosity. Titration models can involve extremely high fluid:rock ratios that represent unrealistically high instantaneous fluid:rock unless reaction is taking place in a fluid-filled cavity. Nevertheless, such models can provide important insights into what are the most important variables.
We also carried “flush” experiments in which a mass of rock is specified (and which changes due to additions or reductions due to fluid-rock reaction) and a specified mass of hydrothermal fluid is passed through the rock in a series of increments displacing an equivalent volume of existing “pore” fluid (Bethke, 1996). We can approximate porosity by specifying the volume of rock in the initial stage of the calculation and by choosing an appropriate mass (volume) of fluid. Porosity is therefore independent of the total amount of fluid flushed through the model rock (see Bethke, 1996, for further discussion). Thus, this type of calculation approximates more closely the processes of nature.
The current version of GWB uses a database that is in part derived from the various SUPCRT compilations (see Bethke, 1996 and references therein). Thermodynamic properties for various aqueous Pt species are derived from the compilation of Sassani and Shock (1998).
Choice of Hydrothermal Fluid Composition
A reconnaissance for fluid inclusions as part of this study has failed to provide suitable material for thermometric analysis. Thus, we have little direct information regarding the temperature and composition of waters involved in hydrothermal alteration. Palaeoclimatic data cited earlier suggest that at least three distinct fluid types may have participated in the Tertiary alteration event or events. Meteoric water was available during times of emergence, but marine water and evaporated seawater are alternatives. Other fluid types such as magmatic, metamorphic (due to devolatisation reactions) and basinal brines are unlikely given the geological setting. As an approximation of meteoric water we have used the composition of present day groundwaters. Stanger (1984) has documented two main groundwater compositions: slightly alkaline water found in gabbros and basalts of the ophiolite (“crustal groundwater”) and hyperalkaline water found in serpentinised ultramafic rocks. Thus, calculations were performed with four fluid compositions (Table 6).
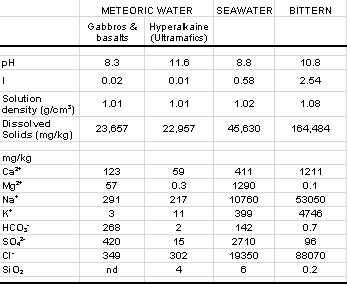 |
| Table 6: Representative hydrothermal fluid compositions (from Stanger, 1984; Stanger & Neal, 1984). |
We surmise that maximum temperatures in the listwaenite bodies during the Tertiary were less than 65¼C based on the studies of the Rusayl Formation (see above). Stanger (1985) estimated that the formation of niccolite and maucherite in the altered ophiolite occurred at a temperature of 40 - 45¼C based on Ni:As ratio. This conclusion was based, however, on extrapolation of experimental data from 200¼C and furthermore, there is textural evidence of disequilibrium between the two minerals (Stanger, 1985). Present day hyperalkaline water is inferred to have circulated to depths of 700m based on anomalously hot temperatures at springs, which are as much as 8¼C hotter than ambient, and a geothermal gradient of 20¼C/km (Stanger, 1984).
A significant uncertainty is the oxidation state of the waters, which was arbitrarily assumed to be in equilibrium with atmospheric oxygen. Whether Pt is carried as a chloride complex or as an hydroxyl complex, solubility is strongly dependent on oxidation state.
Possible Sources of Pt and Model Rock Compositions
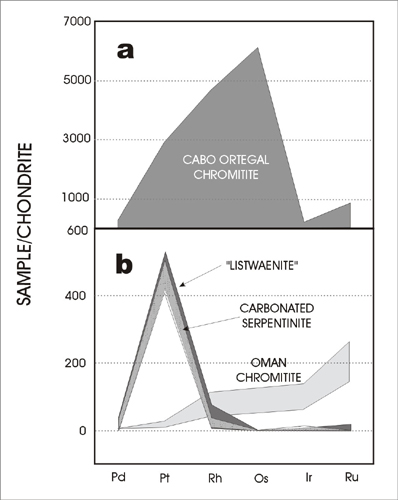
Table 5 lists a compilation of published and our new PGE analyses of rocks from Oman. Enriched Pt rocks include chromite pods of the “mantle sequence” harzburgites, magmatic sulphides from ophiolite gabbro and serpentinite. Chromite pods in the Semail ophiolite are hosted by the basal harzburgite and commonly contain elevated Ir, Ru and Rh but generally low Pt and Pd (Page et al., 1982). Magmatic silicates in these pods are often partially to completely replaced by serpentine minerals, indicating passage of late hydrothermal (metamorphic) fluid. In other environments, chromites can be considerably enriched in Pt and Pd relative to Ir, Ru and Rh (e.g. Spain, Moreno et al., 2001; Fig. 5). It is possible, therefore, that the PGE patterns of Oman chromites reported by Page et al., (1982) represent Pt and Pd depletion by hydrothermal fluids.
| Figure 5: Spider diagram showing the ranges of platinum-group metals in various lithologies, including chromite from Oman and Spain (Moreno et al., 2001). |
Another possible source lies in magmatic sulphide concentrations in the gabbroic portion of the ophiolite (e.g. Lorand & Juteau, 2000). Whole-rock PGE concentrations of economic levels have not been located so far, but as much as 120 ppb Pt has been detected in a sulphide separate (Table 5). These PGE-enriched sulphides are somewhat rare, certainly in comparison to chromitite pods. Extreme Pt-enrichment has been recorded in a single sample of pale-coloured serpentinite reported by LeBlanc et al. (1991; Table 5). There has not been a systematic attempt to find similar Pt-rich rocks in Oman and whether this represents a possible source rock or indeed an economic host in its own right remains to be seen.
Thus our models used serpentinite, chromitite, gabbro and sulphidic gabbro (Table 7) in an attempt to understand what makes a good Pt source rock in this environment. Pt minerals used in the calculations were metallic Pt and PtS (which is stable depends mainly on the sulphur concentrations adopted and oxidation state).
 |
| Table 7: Modal composition of rocks used in geochemical modelling. |